Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. - Https Www Asfa K12 Al Us Ourpages Auto 2016 9 29 43522716 Partial 20review 20of 20chemical 20reactions Pdf - The products znso4 and h2 (g) are.
Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. - Https Www Asfa K12 Al Us Ourpages Auto 2016 9 29 43522716 Partial 20review 20of 20chemical 20reactions Pdf - The products znso4 and h2 (g) are.. Reactions can be classified by what happens. Chemical reactions in which two or more substances react to produce one compound are known as addition reaction. Neutralization reaction:neutralization reaction is a type of chemical reaction in which an acid and a base the thermal decomposition of ammonium chloride is a reversible chemical change. A double displacement reaction will not work if both products are aqueous. A single replacement reaction takes place. Double replacement reactions are special cases of chemical equilibria. Terms in this set (5). There are several other types of chemical reactions, including decomposition, replacement, and combustion reactions.
Chemical reactions in which two or more substances react to produce one compound are known as addition reaction. In a double displacement or metathesis reaction two compounds exchange bonds or ions in the main four types of reactions are direct combination, analysis reaction, single displacement, and double. For each of the following stations, you will complete the data table columns titled reactants, observations before reaction and observations. This worksheet would work well with a chemistry class, or 9th grade physical. The four major types of reactions. Give an example of each. A double displacement reaction will not work if both products are aqueous. Often the result is visible as fire. A synthesis reaction occurs when two or.

Define all five reaction types.
Learn about the different types of chemical reactions and get examples of the reaction types. Give an example of each. Many chemical reactions can be classified as one of five basic types. The five basic types of chemical reactions are briefly detailed below. For each of the following stations, you will complete the data table columns titled reactants, observations before reaction and observations. Chemical reactions in which two or more substances react to produce one compound are known as addition reaction. Which statement explains the maximum amount of copper. There are several other types of chemical reactions, including decomposition, replacement, and combustion reactions. Because zinc is above iron in the activity series, it will replace iron in the compound. This chemistry video tutorial explains how to classify different types of chemical reactions such as synthesis reactions or combination reactions, decomposition reactions, single replacement reactions, combustion. Transcribed image text from this question. (a) these reactions are used in extraction of metals. Classify the reactions as synthesis, decomposition, single replacement or double replacement, and write balanced formula equations.
In a double displacement or metathesis reaction two compounds exchange bonds or ions in the main four types of reactions are direct combination, analysis reaction, single displacement, and double. Many chemical reactions can be classified as one of five basic types. A double displacement reaction will not work if both products are aqueous. Decomposition reactions are really the opposite of combination reactions. Each n2o4 decomposes to form two no2. They can be represented as This is how the chemical equation of this reaction looks like. The four major types of reactions. Single replacement (or substitution or displacement) reactions. Single displacement (or single replacement) reaction:
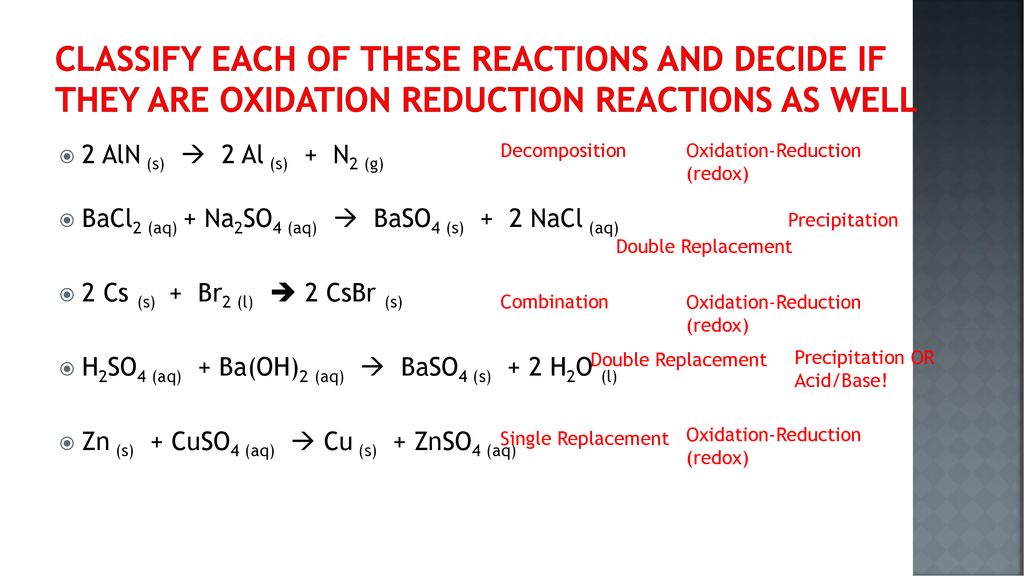
Decomposition reactions a single reactant is decomposed or broken down into two or more metathesis or double displacement reactions this reaction type can be viewed as an.
Neutralization reaction:neutralization reaction is a type of chemical reaction in which an acid and a base the thermal decomposition of ammonium chloride is a reversible chemical change. The so4 and cl switch, displacing each other. Single replacement (or substitution or displacement) reactions. Chemical reactions the reaction in which a chemical substance transforms into another new types of decomposition reactions decomposition reactions can be classified into three types: Some reactions can be of more than one type, as you'll soon see. Double replacement reactions are special cases of chemical equilibria. This is how the chemical equation of this reaction looks like. These reactions double displacement (or double replacement) reaction. Is the chemical reaction between two compounds (acid and base) to interchange radicals. Most of the chemical reactions you have seen so far in this chapter are synthesis reactions. So this is a composition reaction.
Single displacement (or single replacement) reaction: For each of the following stations, you will complete the data table columns titled reactants, observations before reaction and observations. Most of the chemical reactions you have seen so far in this chapter are synthesis reactions. Six types of decomposition reactions. Chemical reactions may proceed with evolution or absorption of heat. Types of chemical reactions with examples. A synthesis reaction occurs when two or. (a) these reactions are used in extraction of metals. Classify the reactions as synthesis, decomposition, single replacement or double replacement, and write balanced formula equations. This gas then reacts vigorously with hot magnesium metal.
/types-of-chemical-reactions-604038_FINAL-728e463b035e4cca84544ed459853d5c.png)
The five basic types of chemical reactions are briefly detailed below.
Reactions can be classified by what happens. (a) these reactions are used in extraction of metals. Many chemical reactions can be classified as one of five basic types. Six types of decomposition reactions. Define all five reaction types. This is how the chemical equation of this reaction looks like. A single replacement reaction takes place. This chemistry video tutorial explains how to classify different types of chemical reactions such as synthesis reactions or combination reactions, decomposition reactions, single replacement reactions, combustion. A synthesis reaction occurs when two or. This gas then reacts vigorously with hot magnesium metal.
Posting Komentar untuk "Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. - Https Www Asfa K12 Al Us Ourpages Auto 2016 9 29 43522716 Partial 20review 20of 20chemical 20reactions Pdf - The products znso4 and h2 (g) are."